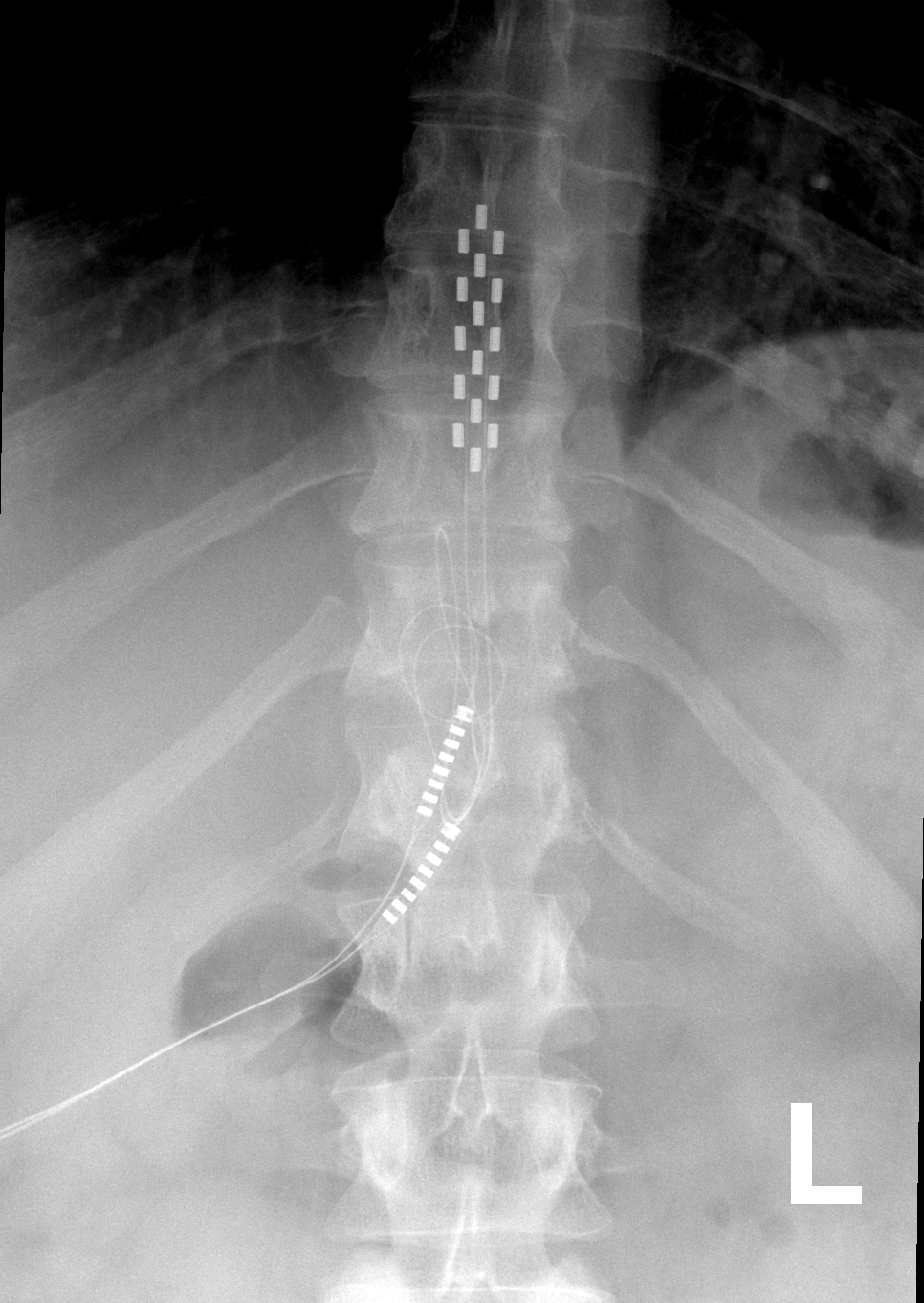
Senza Spinal Cord Stimulation System – P130022/S039
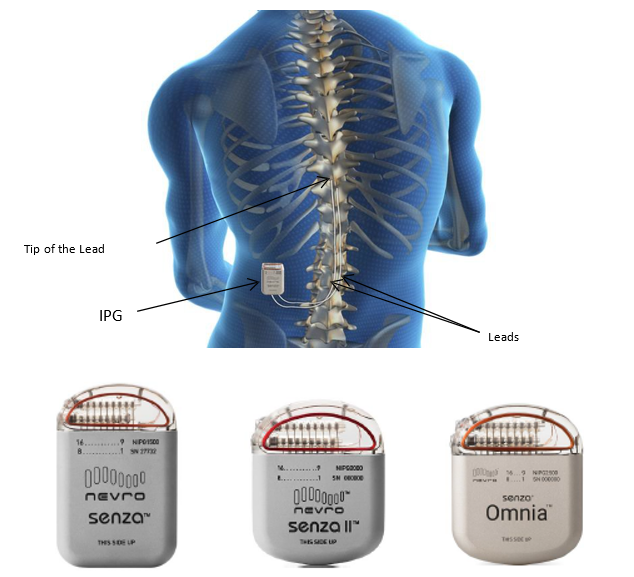
Senza Spinal Cord Stimulation System – P130022/S039
The Senza, Senza II, and Senza Omnia are implanted, rechargeable Spinal Cord Stimulation systems to treat chronic pain in a patient’s trunk or limbs that is difficult to manage.

FDA Approves First Smart Spinal Cord Stimulation Trial System
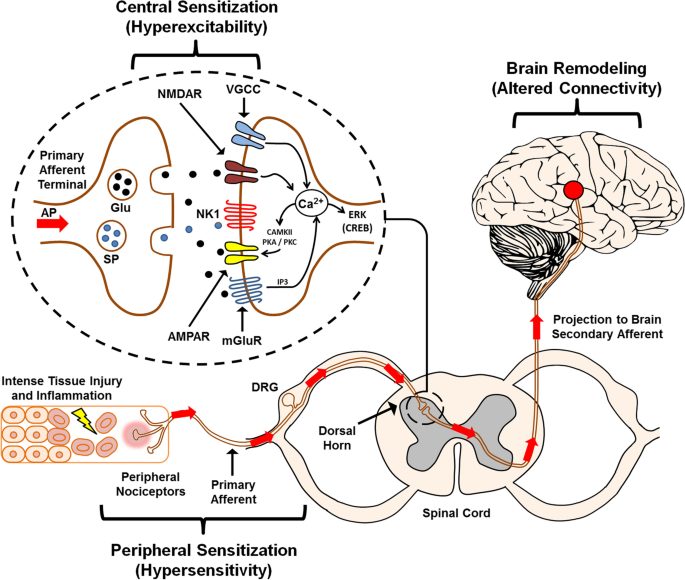
Advances in targeting central sensitization and brain plasticity in chronic pain, The Egyptian Journal of Neurology, Psychiatry and Neurosurgery

Nevro Corp. - Nevro Announces FDA Approval for Expanded Labeling for its 10 kHz High Frequency Spinal Cord Stimulation System for Treatment of Non-Surgical Refractory Back Pain (NSRBP)

Spinal Cord Stimulator Procedure, Recovery, & Restrictions

Nevro receives CE mark for full-body MRI conditional labelling with the Senza system - NeuroNews International

Advanced Temporally‐Spatially Precise Technologies for On‐Demand Neurological Disorder Intervention - Chen - 2023 - Advanced Science - Wiley Online Library

St. Jude Medical™ Nicht sichtbares Testsystem für SCS
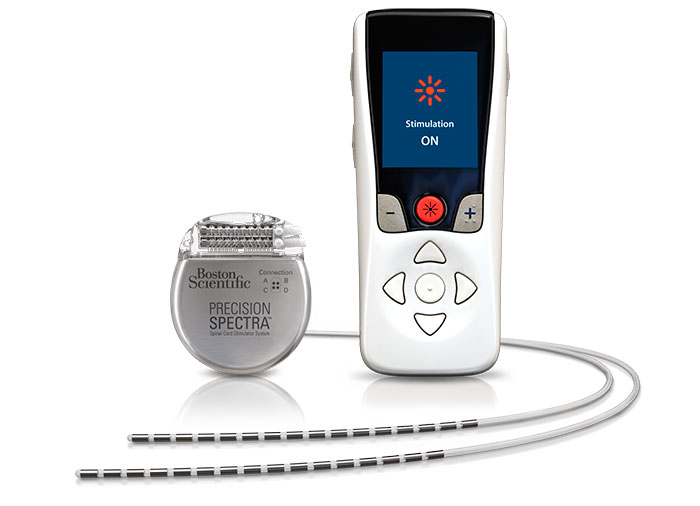
Spinal Cord Stimulation Trial

Spinal cord injury: time to move - The Lancet