FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood

FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.

Mouse Archives - Inside Precision Medicine

ASCA: A new program for biocompatibility testing, NAMSA posted on the topic

FDA Approves CareTaker® Wireless Remote Patient Monitor For Continuous Non-Invasive Blood Pressure (cNIBP) and Heart Rate Monitoring using patented Finger Cuff Technology

Press Releases — Nanowear

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous Blood Pressure Monitoring and Hypertension Diagnostic Management: SimpleSense-BP, News
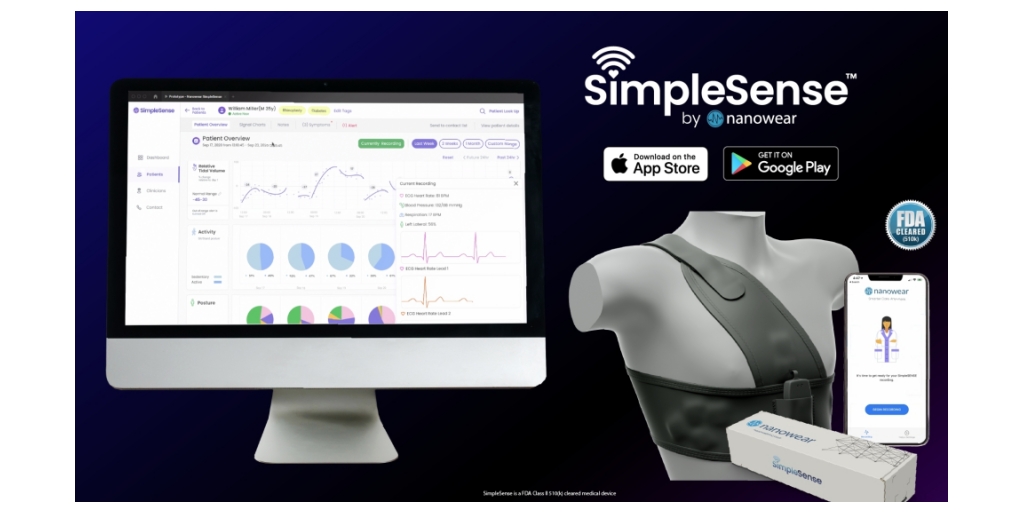
Nanowear Receives FDA 510(k) Platform Clearance to Implement Forthcoming AI-based Diagnostics in its Closed Loop Hospital-at-Home Network

DNAnexus Wins Five-Year, $20M Contract to Power precisionFDA Platform

Nanowear SimpleSense Wearable Receives FDA Clearance

Ep 22 Introducing Nanotechnology to improve patient outcomes - Venk Varadan - Key Tech
Precision Medicine Patient Care Articles & News - Inside Precision Medicine

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous Blood Pressure Monitoring and Hypertension Diagnostic Management: SimpleSense-BP
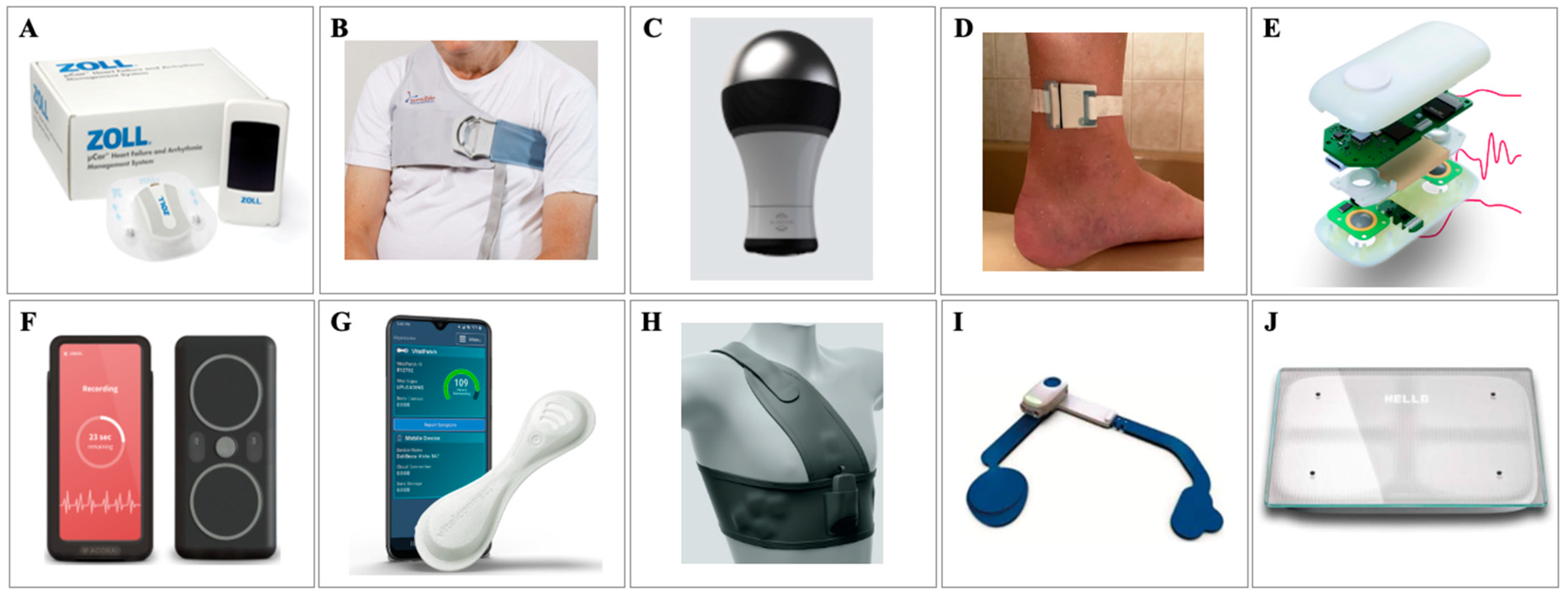
JCM, Free Full-Text


