Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From

Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From

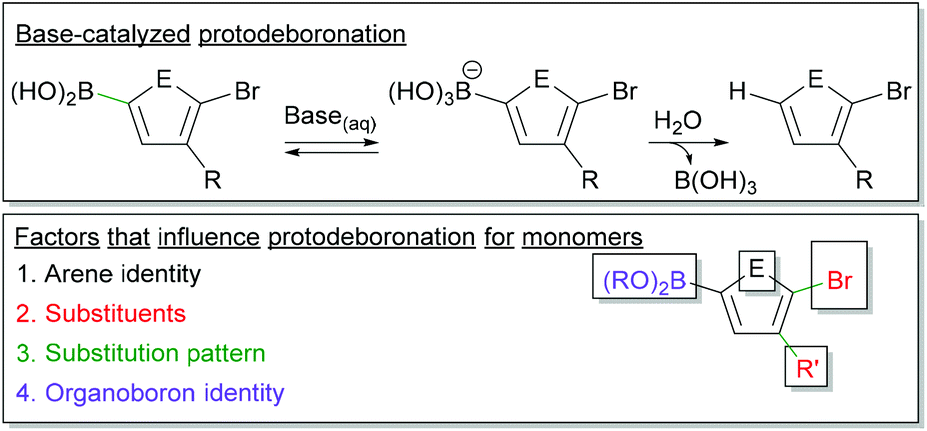
Figure 11 from Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion.

Non-innocent electrophiles go beyond Research Communities by Springer Nature
Molecules, Free Full-Text
Pairing Suzuki–Miyaura cross-coupling and catalyst transfer polymerization - Polymer Chemistry (RSC Publishing) DOI:10.1039/D0PY01507E
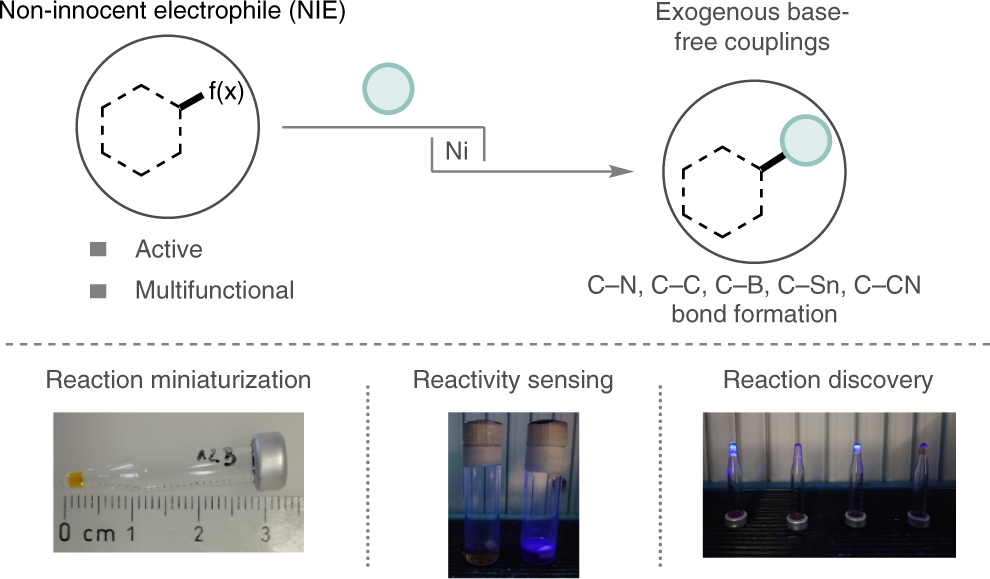
Non-innocent electrophiles unlock exogenous base-free coupling reactions
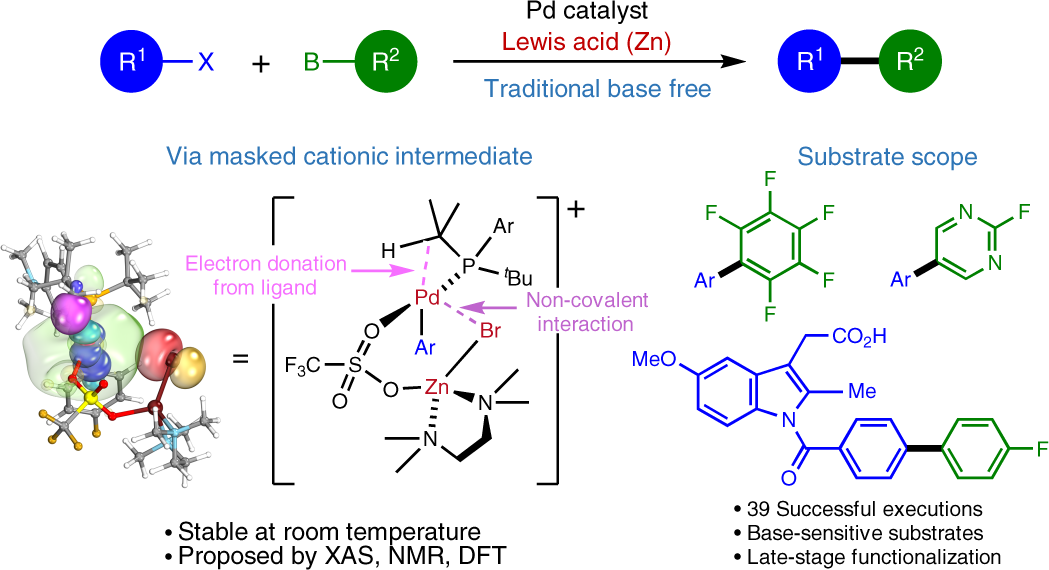
Lewis acid-mediated Suzuki–Miyaura cross-coupling reaction

Base-free nickel-catalysed decarbonylative Suzuki-Miyaura coupling of acid fluorides. - Abstract - Europe PMC

PDF] A mechanistic proposal for the protodeboronation of neat boronic acids: boronic acid mediated reaction in the solid state.

Figure 11 from Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion.

Base-catalyzed Aryl-B(OH)2 Protodeboronation Revisited: from Concerted Proton-Transfer to Liberation of a Transient Arylanion. - CORE

Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion

Development and Molecular Understanding of a Pd‐Catalyzed Cyanation of Aryl Boronic Acids Enabled by High‐Throughput Experimentation and Data Analysis - De Jesus Silva - 2021 - Helvetica Chimica Acta - Wiley Online Library

Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion

PDF] Protodeboronation of Heteroaromatic, Vinyl, and Cyclopropyl Boronic Acids: pH-Rate Profiles, Autocatalysis, and Disproportionation.

Base-Promoted Protodeboronation of 2,6-Disubstituted Arylboronic Acids